Answer:
W = 0 J
Step-by-step explanation:
The amount of work done by gas at constant pressure is given by the following formula:
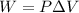
where,
W = Work done by the gas
P = Pressure of the gas
ΔV = Change in the volume of the gas
Since the volume of the gas is constant. Therefore, there is no change in the volume of the gas:
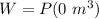
W = 0 J