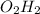Answer:
E.F= OH
M.F=
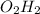
Step-by-step explanation:
Empirical Formula
Step 1: Calculate mols of each element
0.44gH(1 mol H/ 1.008g H)= 0.4365 mol H
*note leave extra sig figs for calculations
6.92gO(1 mol O/ 16 g O)=0.4325 mol O
Step 2: Identify which is the smallest mol
*in this case 0.4365 mol H> 0.4325 mol O so we will use 0.4325 mol O
Step 3: Divide above calculations by the smallest mol
0.4325 mol O/0.4325= 1
0.4365 mol H/0.4325= 1.009 *rounds to 1
Step 4: use calculations as subscripts
Oxygen = 1 so the subscript will 1 (O)
Hydrogen = 1 so the subscript will 1 (H)
making E.F= OH
Molecular Formula:
Step 1: identify molecular mass and mass from the E.F
the molecular mass is given 34.00g/mol
the mass of the E.F is
(oxygen mass from periodic table)+(hydrogen mass from periodic table)
16+1.008= 17.008
Step 2:Divide the molecular mass by the mass given by the emipirical formula.
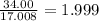 round to 2
round to 2
Step 3:Multiply the empirical formula (the subscripts) by this number to get the molecular formula. ANSWER: M.F=2(OH)-