Answer:
Final pressure = 362.7 Pa
Step-by-step explanation:
Given that,
Initial volume, V₁ = 930 ml
Initial pressure P₁ = 156 Pa
Final volume, V₂ = 400 mL
We need to find the final pressure. We know that the relation between volume and pressure is inverse i.e.
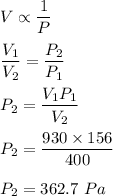
So, the final pressure is equal to 362.7 Pa.