Answer:
Q = 768.47 J
Step-by-step explanation:
Given that,
Mass of the metal, m = 25 g
Initial temperature, T₁ = 21.0 ºC
Final temperature, T₂ = 80.0 ºC
The specific heat of the metal is 0.521 J/gºC.
We know that the heat released due to the change in temperature is given by :
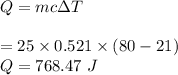
Hence, 768.47 J of heat energy will be needed.