Answer:
0.933 L
Step-by-step explanation:
Since the pressure is the same, we use the equation
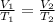
V = Volume
T = Temperature
Since we are given the temperature in Celsius, we need to convert it to Kelvin by adding 273:
-55.0 + 273 = 218
40.0 + 273 = 313
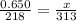
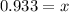
The gas will occupy a volume of 0.933 L.
(Side note - If the temperature increases, the gas will want to expand, leading to a higher volume.)