Answer:
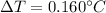
Step-by-step explanation:
Hello there!
In this case, according to the following equation for the calculation of heat in this calorimetry problem:
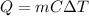
It is possible for us to calculate to calculate the change in temperature for this process by solving for DT in the aforementioned equation:
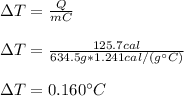
Best regards!