Answer:
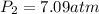
Step-by-step explanation:
Hello!
In this case, given the change in volume and pressure of the gas, it is possible for us to recall the Boyle's law as way to understand the inversely proportional relationship between pressure and volume:
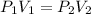
Thus, when solving for the final pressure, P2, given the initial pressure and volume and the final volume, we obtain:
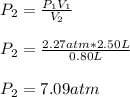
Best regards!