Given :
A cylinder of Krypton has contains 17 L of Ar at 22.8 atm and 112 degrees Celsius.
To Find :
How many moles are in the cylinder.
Solution :
We know, by ideal gas equation :
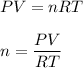
Here, R is gas constant and
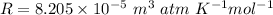
Converting all given in required units and putting in above equation, we get :
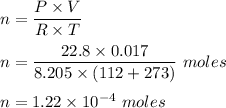
Hence, this is the required solution.