Answer:

Step-by-step explanation:
The molarity of a solution is found by dividing the moles of solute by the liters. of solvent.
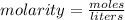
We know the solution has 3.6 moles of potassium chlorine. We know there are 750 milliliters of solvent, but we need to convert this to liters.
- 1 liter is equal to 1000 milliliters. Set up a proportion.
- Multiply by 750 mL and the units of mL will cancel.
Now we know the moles and liters, so we can calculate molarity.
- moles= 3.6 mol KCl
- liters= 0.75 L
Substitute these values into the formula.
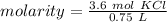
Divide.
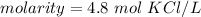
- 1 mole per liter is equal to 1 molar.
- Our answer of 4.8 moles of potassium chloride per liter is equal to 4.8 M KCl
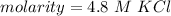
The molarity of the solution is 4.8 M KCl.