Answer:
2.5 moles of ions
Step-by-step explanation:
In order to solve this problem, first we need to determine how many moles of ions are there in one mol of aluminum sulfate:
- 1 Al₂(SO₄)₃ mol contains 2 Al⁺³ moles and 3 SO₄⁻³ moles, meaning that in total it contains 5 moles of ions.
With the above information in mind we can proceed to calculate how many moles of ions would there be in 0.5 moles of Al₂(SO₄)₃:
- 0.5 mol Al₂(SO₄)₃ *
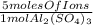 = 2.5 moles of ions
= 2.5 moles of ions