Answer:
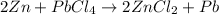
Step-by-step explanation:
Hello there!
In this case, when referring to single replacement reactions, it is crucial for us to figure out the formula of the starting reactants; thus, we know zinc is Zn and lead (IV) chloride is PbCl₄. In such a way, the reaction proceeds as follows:
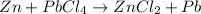
Which must be balanced as shown below:
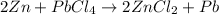
Regards!