Answer: There are 0.854 moles of
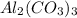 present in 200 g of
present in 200 g of
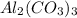 .
.
Step-by-step explanation:
Given: Mass of
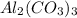 = 200 g
= 200 g
As number of moles is the mass of substance divided by its molar mass.
Molar mass of
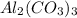 is 233.99 g/mol.
is 233.99 g/mol.
Therefore, moles of
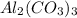 is calculated as follows.
is calculated as follows.
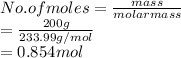
Thus, we can conclude that there are 0.854 moles of
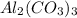 present in 200 g of
present in 200 g of
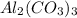 .
.