Answer:
K = 9.620 × 10⁻⁶
Step-by-step explanation:
From the given information:
Temperature T= 6° C
= (273 + 6)K
= 279 K
The correct and well presentation of the reactions are:
1.
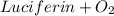 ⇆ oxyluciferin + light ΔG₁°
⇆ oxyluciferin + light ΔG₁°
2. ATP ⇄ AMP + PP
 ΔG₂° = -31.6 kJ/mol
ΔG₂° = -31.6 kJ/mol
The overall ΔG° = -4.80 kJ/mol
Let's first determine the ΔG₁° for the equation (1)
ΔG° = ΔG₁° + ΔG₂°
- ΔG₁° = - ΔG° + ΔG₂°
ΔG₁° = ΔG° - ΔG₂°
ΔG₁° = ( -4.80 - (-31.6) ) kJ/mol
ΔG₁° = 26.8 kJ/mol
Using the formula:
ΔG° = -RTIn K
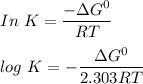
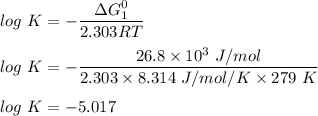
K = antilog (-5.017)
K = 9.620 × 10⁻⁶