Answer: The molarity of 8 grams of an aqueous solution of sodium hydroxide in 2 liters of solute is 0.1 M
Step-by-step explanation:
Given: Mass of solute = 8 g
Volume of solution = 2 L
Molar mass of NaOH is 40 g/mol.
Number of moles of NaOH are calculated as follows.
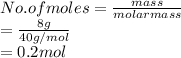
As molarity is the number of moles of solute present in a liter of solution. Therefore, molarity of given solution is calculated as follows.
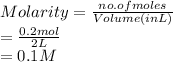
Thus, we can conclude that the molarity of 8 grams of an aqueous solution of sodium hydroxide in 2 liters of solute is 0.1 M.