Answer:
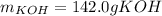
Step-by-step explanation:
Hello there!
In this case, according to the following chemical reaction we found on goo gle as it was not given:
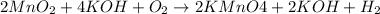
Whereas we can see a 2:4 mole ratio of potassium permanganate product to potassium hydroxide reactant with molar masses of 158.03 g/mol and 54.11 g/mol respectively. In such a way, by developing the following stoichiometric setup, we obtain the mass of KOH to start with:
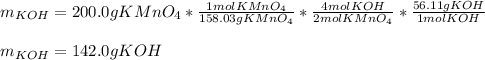
Best regards!