Answer: An ionic bond is a bond that forms between ions with opposite charges.
Step-by-step explanation:
A chemical bond formed by the transfer of electrons from one atom to another is called an ionic bond.
For example, sodium has electronic distribution as 2, 8, 1 and chlorine has electronic distribution as 2, 8, 7.
In order to attain stability, sodium needs to lose 1 electron and chlorine needs to gain one electron. Therefore, sodium will transfer its one valence electron (forming
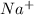 ion) to chlorine atom (forming
ion) to chlorine atom (forming
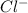 ion) which leads to the formation of NaCl compound.
ion) which leads to the formation of NaCl compound.
Thus, we can conclude that an ionic bond is a bond that forms between ions with opposite charges.