Answer:
1)Molarity of 0.5 Moles of Sodium Chloride in 0.05 Liters of solution is 10 M.
2)Molarity of 734 grams of lithium sulfate are dissolved to make 2500 mL of solution is 2.68 M.
3)Molarity of a solution is made by adding 83 grams of sodium hydroxide to 750 mL of water is 2.77 M.
4)Molarity of a solution that contains .500 mol HC₂H₃O₂ in 0.125 kg H₂O is 4 M.
5)Molarity of a solution that contains 63.0 g HNO₃ in 0.500 kg H₂O is 2 M.
6)The mass of water required to form 3.0 M solution by dissolving 0.5kg of C₂H₅OH is 3.62 kg.
Step-by-step explanation:
Molarity is given as
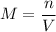
Here
- n is number of moles
- V is the volume of the solution in liters
Using this all the values are calculated as follows:
1)Molarity of 0.5 Moles of Sodium Chloride in 0.05 Liters of solution is
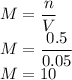
So the molarity is 10 M.
2)Molarity of 734 grams of lithium sulfate are dissolved to make 2500 mL of solution is
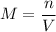
Here n is calculated as follows:
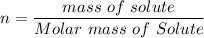
Here the molar mass of lithium sulphate is as follows:
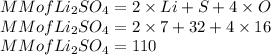
So n is
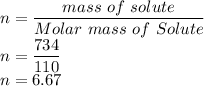
V is given as
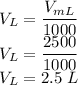
So the molarity is given as

So the molarity is 2.68 M.
3)Molarity of a solution is made by adding 83 grams of sodium hydroxide to 750 mL of water is
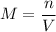
Here n is calculated as follows:
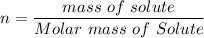
Here the molar mass of Sodium hydroxide is as follows:
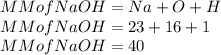
So n is
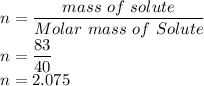
V is given as
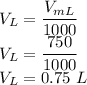
So the molarity is given as
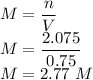
So the molarity is 2.77 M.
4)Molarity of a solution that contains .500 mol HC₂H₃O₂ in 0.125 kg H₂O is
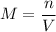
V is given as
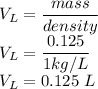
So the molarity is given as
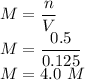
So the molarity is 4.00 M.
5)Molarity of a solution that contains 63.0 g HNO₃ in 0.500 kg H₂O is
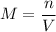
Here n is calculated as follows:
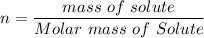
Here the molar mass of HNO₃ is as follows:
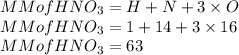
So n is
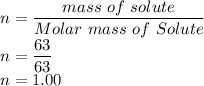
V is given as
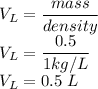
So the molarity is given as
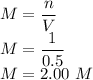
So the molarity is 2.00 M.
6)Mass of water must be used to dissolve 0.500 kg C₂H₅OH to prepare a 3.00 m solution is calculated as follows
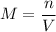
Here n is calculated as follows:
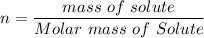
Here the molar mass of C₂H₅OH is as follows:
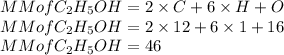
So n is
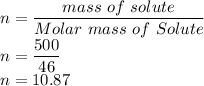
V required is given as
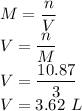
So the mass of water is given as
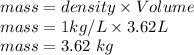
So the mass of water required to form 3.0 M solution by dissolving 0.5kg of C₂H₅OH is 3.62 kg.