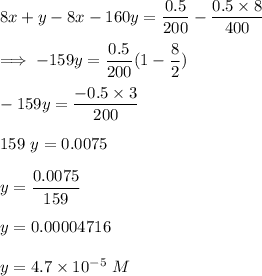Answer:
Step-by-step explanation:
From the given information:
At wavelength = 270 nm
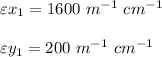
At 270 nm
Suppose x is said to be the solution for the concentration of x and y to be the solution for the concentration of y;
Then:
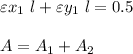
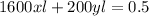
Divide both sides by 200
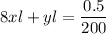
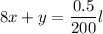
Use l = 1cm (i.e the standard length)
Then;
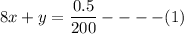
For 540 nm:
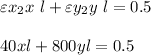
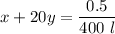
since l = 1
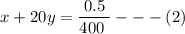
Equating both (1) and (2) together, we have: