Answer:
Concentration of ethanol required = 48.476 M
Step-by-step explanation:
Given that:
the absorption intensity = 1.00
Molarity of ethanol = 1M
NMR instrument used = 160 MHz
Temperature used = 300 K
The required concentration of ethanol can be determined as follows:
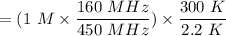
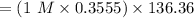
= 48.476 M