Answer:
The age of the rock is 2.23x10⁹ years.
Step-by-step explanation:
The age of the rock can be calculated using the following equation:
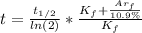 (1)
(1)
Where:
t: is the age of the rock =?
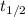 : is the half-life of ⁴⁰K = 1.248x10⁹ years
: is the half-life of ⁴⁰K = 1.248x10⁹ years
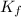 : is the quantity of ⁴⁰K in the sample = 281.8x10⁻⁹ mol
: is the quantity of ⁴⁰K in the sample = 281.8x10⁻⁹ mol
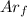 : is the quantity of ⁴⁰Ar in the sample = 7.25x10⁻⁹ mol
: is the quantity of ⁴⁰Ar in the sample = 7.25x10⁻⁹ mol
10.9%: is the percent of ⁴⁰Ar production by the decay of ⁴⁰K
By entering the above values into equation (1) we have:
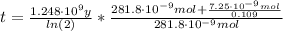
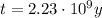
Therefore, the age of the rock is 2.23x10⁹ years.
I hope it helps you!