Answer:
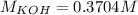
Step-by-step explanation:
Hello there!
In this case, since the titration of bases when using monoprotic acids like KHP, occurs in a 1:1 mole ratio, it is possible to use the following equation, because at the endpoint the moles of the KHP and KOH get equal:
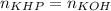
In such a way, we first calculate the moles of KOH given the mass and molar mass of KHP:
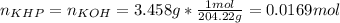
Next, since we have the volume of KOH, we first take it to liters (0.04571 L) to that we obtain the following concentration:
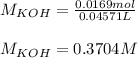
Regards!