Answer:
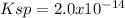
Step-by-step explanation:
Hello there!
In this case, given the solubilization of cadmium (II) hydroxide:
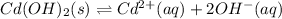
The solubility product can be set up as follows:
![Ksp=[Cd^(2+)][OH^-]^2](https://img.qammunity.org/2022/formulas/chemistry/high-school/lt92u6fhcmhln8iy9qyq5bot40ii6hvwi7.png)
Now, since we know the concentration of cadmium (II) ions at equilibrium and the mole ratio of these ions to the hydroxide ions is 1:2, we infer that the concentration of the latter at equilibrium is 3.5x10⁻⁵ M. In such a way, the resulting Ksp turns out to be:

Regards!