Answer:
1.75 moles are contained in 500 mL of a 3.5 M NaOH solution.
Step-by-step explanation:
Molar concentration is a measure of the concentration of a solute in a solution, be it some molecular, ionic or atomic species and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution.
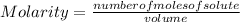
Molarity is expressed in units
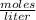 .
.
In this case:
- molarity= 3.5 M
- number of moles of solute= ?
- volume= 500 mL= 0.5 L
Replacing:
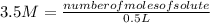
Solving:
number of moles of solute= 3.5 M*0.5 L
number of moles of solute= 1.75 moles
1.75 moles are contained in 500 mL of a 3.5 M NaOH solution.