Answer:
No, no precipitate is formed.
Step-by-step explanation:
Hello there!
In this case, since the reaction between ammonium sulfide and aluminum nitrate is:
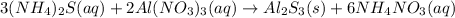
In such a way, we can calculate the concentration of aluminum and sulfide ions in the solution as shown below, and considering that the final total volume is 140.00 mL:
![[Al^3^+]=(120.00mL*0.0082M)/(140.00mL)=0.00703M](https://img.qammunity.org/2022/formulas/chemistry/college/2tzoq924cyx32lw8bn43zcn73pwbgg31sx.png)
![[S^2^-]=(20.00mL*0.0090M)/(140.00mL)=0.00129M](https://img.qammunity.org/2022/formulas/chemistry/college/wnc6ga4rx8drfygwo2giego32l9be8o8zi.png)
In such a way, we can calculate the precipitation quotient by:
![Q=[Al^3^+]^2[S^2^-]^3=(0.00703)^2(0.00129)^3=1.05x10^(-13)](https://img.qammunity.org/2022/formulas/chemistry/college/bvj7p01g588r7t1o1t7me1rh8w27em1e1y.png)
Which is smaller than Ksp and meaning that the precipitation does not occur.
Regards!