Answer:
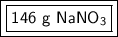
Step-by-step explanation:
To convert from moles to mass, the molar mass is used. This tells us the grams in 1 mole of a substance.
1. Write Formula
We know the compound is sodium nitrate.
Sodium has an oxidation state of +1 and nitrate's is -1. When they bond, sodium gives an electron to nitrate and the charges balance out. We don't need to add any additional subscripts.
2. Calculate Molar Mass
The molar mass is found on the Periodic Table. It is the same as the atomic mass, but the units are grams per mole. Look up the values for the individual elements.
- Na: 22.9897693 g/mol
- N: 14.007 g/mol
- O: 15.999 g/mol
Notice that in the formula, oxygen has a subscript of 3. We must multiply oxygen's molar mass by 3 before adding the other molar masses.
- O₃: 3(15.999 g/mol)= 47.997 g/mol
- NaNO₃= 22.9897693+14.007+ 47.997 = 84.9937693 g/mol
3. Calculate Mass
Use the molar mass as a ratio.
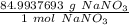
Multiply by the given number of moles: 1.72
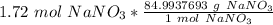
The units of moles of sodium nitrate cancel.
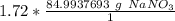
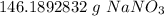
4. Round
The original measurement of moles has 3 significant figures, so our answer must have the same. For the number we found, that is the ones place.
The 1 in the tenths place tells us to leave the 6.
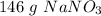
1.72 moles of sodium nitrate has a mass of approximately 146 grams.