Answer:
No, no precipitate is formed.
Step-by-step explanation:
Hello there!
In this case, since the reaction between silver nitrate and sodium acetate is:
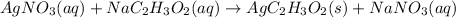
In such a way, we can calculate the concentration of silver and acetate ions in the solution as shown below, and considering that the final total volume is 50.00 mL or 0.0500 L:
![[Ag^+]=(20.00mL*0.077M)/(50.00mL)=0.0308M](https://img.qammunity.org/2022/formulas/chemistry/college/dkkjrn5ujef1xkdscw8v3uv8n1p7p5pejr.png)
![[C_2H_3O_2^-]=(30.00mL*0.043M)/(50.00mL)=0.0258M](https://img.qammunity.org/2022/formulas/chemistry/college/b05895ksoc0w8dadu0n47shi5ra0n7xobr.png)
In such a way, we can calculate the precipitation quotient by:
![Q=[Ag^+][C_2H_3O_2^-]=0.0308*0.0258=7.95x10^(-4)](https://img.qammunity.org/2022/formulas/chemistry/college/u3haqmvalgh37kyjrzm59ow7llwki1f5sa.png)
Which is smaller than Ksp and meaning that the precipitation does not occur.
Regards!