Answer:
Solution A
Step-by-step explanation:
Solution A
1 mole of MgO = 24 + 16 = 40 g
? moles of MgO = 135.75g
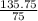 = 1.81 moles
= 1.81 moles
1.81 moles of MgO is in 2 liters of water
? moles of MgO is in 1 liter of water
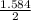 = 0.905moles/liter or 0.905M
= 0.905moles/liter or 0.905M
Solution B
1 mole of CaCl₂ = 40 + 35.5 = 75.5 g
? moles of CaCl₂ = 119.6g
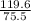 = 1.584moles
= 1.584moles
1.584 moles of MgO is in 2 liters of water
? moles of MgO is in 1 liter of water
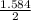 = 0.792moles/liter or 0.792M
= 0.792moles/liter or 0.792M