Answer: There is 0.8 liters (L) of water required to be added to prepare 4800mL of 0.5M NaCl from a 3M NaCl stock solution.
Step-by-step explanation:
Given:
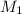 = 0.5 M
= 0.5 M
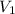 = 4800 mL
= 4800 mL
Convert mL into L as follows.
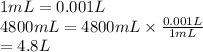
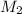 = 3 M
= 3 M
Formula used to calculate the volume of water required as follows.
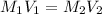
Substitute the values into above formula.
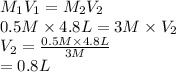
Thus, we can conclude that 0.8 liters (L) of water need to be added to prepare 4800mL of 0.5M NaCl from a 3M NaCl stock solution.