Answer: The pH of solution is 10.
The pOH of the solution is 4.
Step-by-step explanation:
pH is the negative logarithm of concentration of hydrogen ion.
As given concentration of acidic solution is
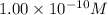 . Therefore, pH of the solution is calculated as follows.
. Therefore, pH of the solution is calculated as follows.
![pH = -log [H^(+)]\\= -log (1.00 * 10^(-10))\\= 10](https://img.qammunity.org/2022/formulas/chemistry/college/u4onbx75auodf9s471vi6enp2dik0uy9vu.png)
The relation between pH and pOH is as follows.
pH + pOH = 14
pOH = 14 - pH
= 14 - 10
= 4
Thus, we an conclude that pH of solution is 10 and pOH of the solution is 4.