Answer:

Step-by-step explanation:
Density measures mass per volume. The volume is
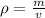
The density of limestone is 2.72 grams per cubic centimeter. We have a piece of limestone with a volume of 8.92 cubic centimeters.
Substitute the values into the formula.
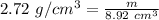
Now we solve for m, the mass, by isolating the variable.
m is being divided by 8.92 cubic centimeters. The inverse of division is multiplication, so we multiply both sides by 8.92 cubic centimeters.
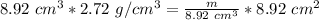
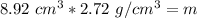
The units of cubic centimeters cancel out.
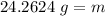
The mass of this piece of limestone is 24.2624 grams.