Answer: The given reaction is a double displacement reaction.
Step-by-step explanation:
A chemical reaction equation in which both positive and negative ions of two different reactants are exchanged then it is called a double displacement reaction.
For example,
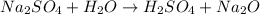
Here, hydrogen ions of
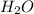 are replaced by the sodium ions of
are replaced by the sodium ions of
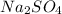 . Also, sulfate ion of
. Also, sulfate ion of
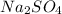 are replaced by oxygen ion of
are replaced by oxygen ion of
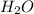 .
.
Thus, we can conclude that the given reaction is a double displacement reaction.