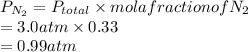Answer: (a) Mole fraction of
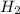 is 0.66.
is 0.66.
Mole fraction of
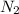 is 0.33
is 0.33
(b) The partial pressure of
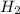 is 1.98 atm.
is 1.98 atm.
The partial pressure of
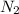 is 0.99 atm.
is 0.99 atm.
(c) The total pressure is 3.0 atm
Step-by-step explanation:
Given: Volume =
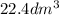 (1
(1
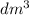 = 1 L) = 22.4 L
= 1 L) = 22.4 L
Moles of
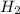 = 2.0 mol
= 2.0 mol
Moles of
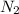 = 1.0 mol
= 1.0 mol
Total moles = (2.0 + 1.0) mol = 3.0 mol
Temperature = 273.15 K
- Now, using ideal gas equation the total pressure is calculated as follows.
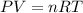
where,
P = pressure
V = volume
n = number of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
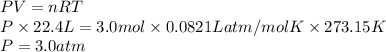
- The mole fractions of each component:
The mole fraction of
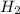 is calculated as follows.
is calculated as follows.
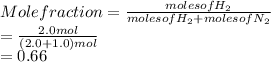
The mole fraction of
 is as follows.
is as follows.
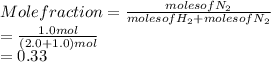
- The partial pressures of each component:
Partial pressure of
 are as follows.
are as follows.
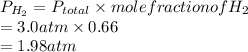
Partial pressure of
 are as follows.
are as follows.