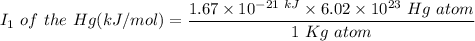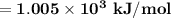Answer:
Step-by-step explanation:
From the given information:
The energy of photons can be determined by using the formula:
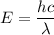
where;
planck's constant (h) =
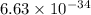
speed oflight (c) =
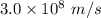
wavelength λ = 58.4 nm
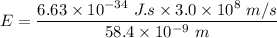
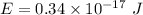
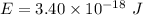
To convert the energy of photon to (eV), we have:
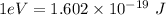
Hence
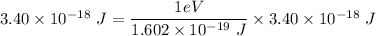
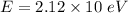
E = 21.2 eV
b)
The equation that illustrates the process relating to the first ionization is:
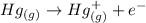
c)
The 1st ionization energy (I.E) of Hg can be calculated as follows:
Recall that:
I.E = Initial energy - Kinetic Energy
I₁ (eV) = 21.2 eV - 10.75 eV
I₁ (eV) = 10.45 eV
Since ;
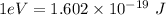
∴
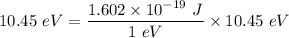
Hence; the 1st ionization energy of Hg atom =
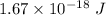
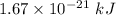
Finally;