Answer:
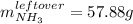
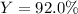
Step-by-step explanation:
Hello there!
In this case, according to the following chemical reaction between iodine monobromide and ammonia:
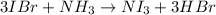
It turns out firstly necessary to identify the limiting reactant, by considering the proper molar masses and the 3:1 and 1:1 mole ratios of iodine monobromide to nitrogen triiodide and ammonia to nitrogen triiodide respectively:
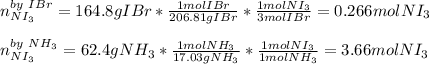
Thus, we conclude that the limiting reactant is IBr as is yields the fewest moles of nitrogen triiodide product. Next, we can calculate the reacted grams of ammonia as the excess reactant:
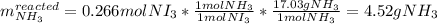
And therefore the leftover of ammonia is:
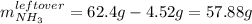
Next, the percent yield is calculated by firstly calculating the theoretical yield of nitrogen triiodide as follows:
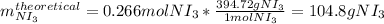
And finally the percent yield by dividing the given actual yield of 96.4 g by the previously computed theoretical yield:
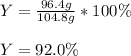
Best regards!