Answer:
Half life is 3.23 hours
Step-by-step explanation:
Given
Decay rate at starting = 1160 decays per minute
Decay rate after 4 hours = 170 decays per minute
As we know know
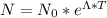
Substituting the given values, we get -
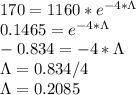
Also
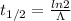
Substituting the given values we get -
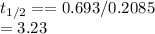 hours
hours