Answer:
97.7 kJ
Step-by-step explanation:
We'll use the formula for specifc heat: Q=mcΔT
Q= heat/energy (Joules), m= mass (grams), c= specific heat (different for each substance and is usually given)
ΔT= final temperature - initial temperature
Let's check our given units and see if they're the correct, here they all are. Let's plug in what we know.
Q=mcΔT
Q=(480)(0.96)(234 - 22)
Q=(480)(0.96)(212)
Q= 97,690 J
Before we submit an answer, let's check out units. We have our answer in Joules, but we want it it kilojoules, so we'll convert it knowing that:
1000 Joules = 1 kilojoule
97,690J x
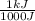 = 97.7kJ
= 97.7kJ