Answer:
The mass of the solution is 140 grams.
Step-by-step explanation:
The mass of the solution is given by the sum of the solute's mass and the solvent's mass:
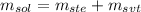
Where:
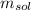 : is the mass of the solution
: is the mass of the solution
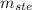 : is the mass of the solute
: is the mass of the solute
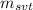 : is the mass of the solvent
: is the mass of the solvent
The solute is the salt (40.0 g) and the solvent is the water (100 g), so:
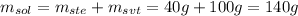
Therefore, the mass of the solution is 140 grams.
I hope it helps you!