Answer:
Avogadro's Law; 15.1L
Step-by-step explanation:
Boyle's Law- Pressure and volume are inversely related, so an increase in pressure= decrease in volume. We see this in the equation P1V1=P2V2.
Charle's Law- Gas expands/increases in volume when temperature increases, so they are proportional. We see this in the formula
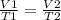 .
.
Guy-Lussac's Law- As temperature increases, pressure increases, so they are proportional. We see this in the formula
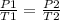 .
.
Avogadro's Law- There are equal amount of volume have an equal amount of moles, so they are proportional. We see this in the formula
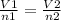 .
.
Plugging in the values based on Avogadro's Law, we'll have:
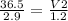
12.6 =
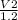
12.6 x 1.2 = V2
15.1L =V2