Answer:
Heat gained by 1.25 grams of ice is 250 joules
Step-by-step explanation:
As we know
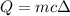 T
T
Where
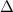 T is the change in temperature
T is the change in temperature
Water freezes at 273.15 K/0 degree Celsius, and it boils at 373.15 K/00 degree Celsius,
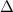 T
T
m is the mass in grams
c is the specific heat of water (ice) = 2.05 joules/gram
Substituting the given values, we get -
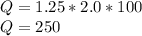 Joules.
Joules.