Answer:
67.3 kPa
Step-by-step explanation:
We're focusing on pressure and volume of gases, so we can use our ideal gas law formula PV=nRT. Everything is constant except pressure and volume, but the values change when moving from city 1 to city 2, so we can say PV city 1= PV city 2.
We can simplify our formula to be P1V1=P2V2. This is specifically Boyle's Law, where if pressure increases (from city 1 to city 2) then volume decreases, thus we can say pressure and volume have an inverse/opposite relationship.
This is an important formula to understand, you can easily memorize it by referring to PV=nRT.
Let's plug in the values we know.
P1V1=P2V2
(101)(6) = (9)(V2)
Now let's solve for V2.
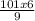 = V2
= V2
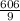 = V2
= V2
67.3 kPa = V2