Answer:
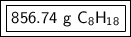
Step-by-step explanation:
To convert from grams to moles, the molar mass is used. These values tells us the grams in 1 mole of a substance. They can be found on the Periodic Table (they are equivalent to the atomic masses, but the units are grams per mole).
We are given the compound C₈H₁₈. Look up the molar masses of the individual elements.
- Carbon (C): 12.011 g/mol
- Hydrogen (H): 1.008 g/mol
Notice there are subscripts that tell us the number of atoms of each element. We must multiply the molar masses by the subscripts.
- C₈: 8(12.011 g/mol)=96.088 g/mol
- H₁₈: 18(1.008 g/mol)=18.144 g/mol
Add these 2 values together to find the molar mass of the whole compound.
- C₈H₁₈: 96.088 g/mol +18.144 g/mol=114.232 g/mol
Use this number as a ratio.
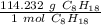
Multiply by the given number of moles: 7.5
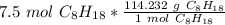
The moles of C₈H₁₈ will cancel each other out.
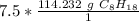
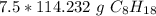
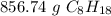
7.5 moles of C₈H₁₈ is equal to 856.74 grams of C₈H₁₈