Answer:
a) number of copper atoms 65 (⁶⁵Cu) is 7.692 10⁶ atoms
b) m_total Cu = 1.585 10⁹ u = 2.632 10⁻¹⁸ kg
Step-by-step explanation:
a) For this exercise let's start by using the radioactive decay ratio
N = N₀
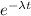 o e - lambda t
o e - lambda t
The half-life time is defined as the time it takes for half of the radioactive (activated) atoms to decay, therefore after two half-lives there are
N = ½ (½ N₀) = ¼ N₀
N₀ = 4 N
in each decay a photon is emitted so we can use a direct rule of proportions. If an atom emits a photon it has Eo = 1,04 Mev, how many photons it has energy E = 10,000 MeV
# _atoms = 1 atom (photon) (E / Eo)
# _atoms = 1 10000 / 1.04
# _atoms = 9615,4 atoms
N₀ = 4 #_atoms
N₀ = 4 9615,4
N₀= 38461.6 atoms
in the exercise indicates that half of the atoms decay in this way and the other half decays directly to the base state of Zinc, so the total number of activated atoms
N_activated = 2 # _atoms
N_activated = 2 38461.6
N_activated = 76923.2
also indicates that 1% = 0.01 of the nuclei is activated by neutron bombardment
N_activated = 0.01 N_total
N_total = N_activated / 0.01
N_total = 76923.2 / 100
N_total = 7.692 10⁶ atoms
so the number of copper atoms 65 (⁶⁵Cu) is 7.692 10⁶
b) the natural abundance of copper is
⁶³Cu 69.17%
⁶⁵Cu 30.83%
Let's use a direct proportion rule. If there are 7.692 10⁶ ⁶⁵Cu that represents 30.83, how much ⁶³Cu is there that represents 69.17%
# _63Cu = 69.17% (7.692 10⁶ / 30.83%)
# _63Cu = 17.258 10⁶ atom ⁶³Cu
the total amount of comatose is
#_total Cu = #_ 65Cu + # _63Cu
#_total Cu = (7.692 + 17.258) 10⁶
#_total Cu = 24.95 10⁶
the atomic mass of copper is m_Cu = 63.546 u
m_total = #_totalCu m_Cu
m_total = 24.95 10⁶ 63,546 u
m_total = 1.585 10⁹ u
let's reduce to kg
m_total Cu = 1.585 10⁹ u (1,66054 10⁻²⁷ kg / 1 u)
m_total Cu = 2.632 10⁻¹⁸ kg