Answer:
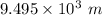
Step-by-step explanation:
From the given information:
Using the equation of Barometric formula as related to density, we have:
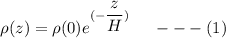
Here;
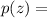 the gas density at altitude z
the gas density at altitude z
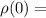 the gas density at sea level
the gas density at sea level
H = height of the scale
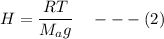
Also;
R represent the gas constant
temperature (T) a= 280 K
g = gravity
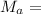 molaar mass of gas; here, the gas is Oxygen:
molaar mass of gas; here, the gas is Oxygen:
∴
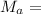 15.99 g/mol
15.99 g/mol
= 15.99 × 10⁻³ kg/mol
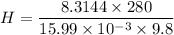
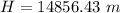
Now we need to figure out how far above sea level the density of oxygen drops to half of what it is at sea level.
This implies that we have to calculate z;
i.e.
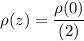
By using the value of H and
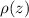 from (1), we have:
from (1), we have:
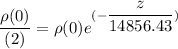
∴
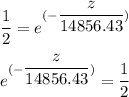
By rearrangement and taking the logarithm of the above equation; we have:
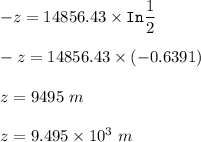
As a result, the oxygen density at
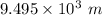 is half of what it is at sea level.
is half of what it is at sea level.