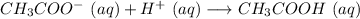Answer:
The answer is "
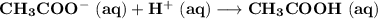 "
"
Step-by-step explanation:
When
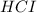 is added in the chemical equation it reacts with sodium acetate so, it will give the following chemical equation:
is added in the chemical equation it reacts with sodium acetate so, it will give the following chemical equation:

In this, the
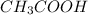 is a weak acid so, it not completely dissociated.
is a weak acid so, it not completely dissociated.
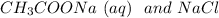 were strong electrolytes they are completely dissociated.
were strong electrolytes they are completely dissociated.
The
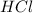 is a strong acid so, it is completely dissociated So, the net ionic equation is:
is a strong acid so, it is completely dissociated So, the net ionic equation is: