Answer: The volume of the sample after the reaction takes place is 29.25 L.
Step-by-step explanation:
The given reaction equation is as follows.
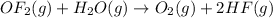
So, moles of product formed are calculated as follows.
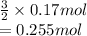
Hence, the given data is as follows.
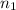 = 0.17 mol,
= 0.17 mol,
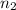 = 0.255 mol
= 0.255 mol
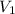 = 19.5 L,
= 19.5 L,
As the temperature and pressure are constant. Hence, formula used to calculate the volume of sample after the reaction is as follows.
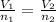
Substitute the values into above formula as follows.
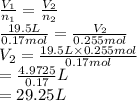
Thus, we can conclude that the volume of the sample after the reaction takes place is 29.25 L.