Answer: The mole ratio of sodium to sodium chloride 2:2.
Step-by-step explanation:
As the given reaction equation is as follows.
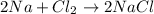
Here, 2 moles of sodium reacts with 1 mole of
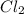 and leads to the formation of 2 moles of NaCl.
and leads to the formation of 2 moles of NaCl.
This means that 2 moles of sodium gives 2 moles of NaCl on reaction with chlorine.
Hence, the ratio of moles of sodium to sodium chloride is 2:2.
Thus, we can conclude that the mole ratio of sodium to sodium chloride 2:2.