Answer: The specific heat of given metal is
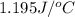 .
.
Step-by-step explanation:
Given: Mass of sample = 22.8 g
Heat energy = 1450 J
Initial temperature =
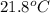
Final temperature =
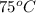
Formula used to calculate the specific heat is as follows.
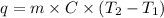
where,
q = heat energy
m = mass of substance or sample
C = specific heat
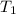 = initial temperature
= initial temperature
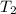 = final temperature
= final temperature
Substitute the values into above formula as follows.
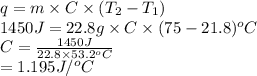
Thus, we can conclude that the specific heat of given metal is
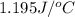 .
.