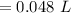Answer:
Option b (V1/T1 = V2/T2) is the right alternative or the new volume will be "0.048 L".
Step-by-step explanation:
The given values are:
Temperature,
T₁ = 25°C
or,
= 298.15 K
T₂ = 50°C
or,
= 323.15 K
Volume,
V₁ = 45 mL
or,
= 0.045 L
V₂ = ?
As we know,
⇒
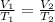
Or,
⇒
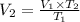
On substituting the values, we get
⇒
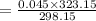
⇒
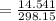
⇒