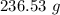Answer:
The right answer is "236.53 g".
Step-by-step explanation:
The given values are:
P = 1.09 atm
V = 186 liters
The reaction will be:
⇒
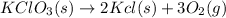
The moles of O₂ will be:
=
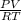
On substituting the values, we get
=
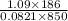
=
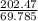
=
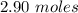
Now,
1 mole O₂ is produced from
=
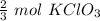
then,
2.90 mole O₂ is produced from 2 mol KClO₃
=
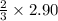
=
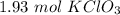
hence,
The number of grans of solid in the engine will be:
=
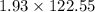
=