Answer:
The final pressure is approximately 0.78 atm
Step-by-step explanation:
The original temperature of the gas, T₁ = 263.0 K
The final temperature of the gas, T₂ = 298.0 K
The original volume of the gas, V₁ = 24.0 liters
The final volume of the gas, V₂ = 35.0 liters
The original pressure of the gas, P₁ = 1.00 atm
Let P₂ represent the final pressure, we get;
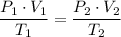
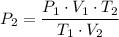
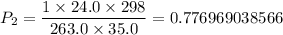
∴ The final pressure P₂ ≈ 0.78 atm.